is dichloromethane a polar solvent Polar aprotic solvents protic nonpolar thf organic acetate dielectric dichloromethane ethyl non dipole table chemistry reactions constants medium master serve
Dichloromethane, also known as methylene chloride, is a versatile solvent that is widely used in various industries, including pharmaceuticals, paints and coatings, and electronics. It is a colorless and volatile liquid with a sweetish aroma and is highly miscible with most organic solvents, making it an ideal solvent for many applications.
The Properties of Dichloromethane
Dichloromethane has a low boiling point of 40°C, which makes it a volatile solvent that is easy to evaporate and has a low residue. This property makes it ideal for use in many industrial applications. Additionally, it is a non-polar solvent that has a low polarity ratio, which makes it an excellent solvent for non-polar compounds.
Dichloromethane has a low viscosity and density, and it does not conduct electricity, making it an excellent solvent for electronic applications. Moreover, it has a high boiling point of about 97°C, which makes it ideal for use in high-temperature applications.
Applications of Dichloromethane
Dichloromethane is an essential solvent in the pharmaceutical industry, where it is used in the manufacture of drugs and as a solvent in the extraction of active pharmaceutical ingredients. It is also used in the production of cosmetics, such as nail polish removers, hair sprays, and lotions.
In the electronics industry, dichloromethane is used to clean and remove residues from electronic components and circuits. It is also used in the production of plastics, fibers, and paints and coatings. Additionally, it is used in the production of adhesives, sealants, and rubber products.
The Safety of Using Dichloromethane
Dichloromethane is a hazardous substance that requires careful handling and disposal. It is toxic when ingested, inhaled, or absorbed through the skin. Therefore, it is essential to handle it in a well-ventilated area, wear protective clothing, and avoid direct contact with skin or eyes.
Apart from being highly flammable, prolonged exposure to dichloromethane can cause various adverse health effects, such as liver damage, nervous system damage, and respiratory problems. Therefore, it is crucial to use it with caution and strictly follow safety guidelines and regulations.
Conclusion
In conclusion, dichloromethane is a useful solvent in various industries due to its low boiling point, non-polarity, and high solvency power. It is widely used in the manufacture of pharmaceuticals, cosmetics, electronics, and paints and coatings. However, it is a hazardous substance that requires careful handling and disposal to avoid adverse health effects on humans and the environment.
 If you are looking to use dichloromethane as a solvent, it is essential to obtain it from a reliable source and use it with care. Contact us for more information on the properties, applications, and safety of dichloromethane.
If you are looking to use dichloromethane as a solvent, it is essential to obtain it from a reliable source and use it with care. Contact us for more information on the properties, applications, and safety of dichloromethane.
 If you are searching about Dichloromethane Formula you’ve came to the right web. We have 5 Pictures about Dichloromethane Formula like Acros Organics AC348460025 Dichloromethane, extra dry, with, Liquid Dichloromethane Solvent, Rs 75 /kilogram Plasma Spray Coats | ID and also Polar Protic? Polar Aprotic? Nonpolar? All About Solvents. Here it is:
If you are searching about Dichloromethane Formula you’ve came to the right web. We have 5 Pictures about Dichloromethane Formula like Acros Organics AC348460025 Dichloromethane, extra dry, with, Liquid Dichloromethane Solvent, Rs 75 /kilogram Plasma Spray Coats | ID and also Polar Protic? Polar Aprotic? Nonpolar? All About Solvents. Here it is:
Dichloromethane Formula
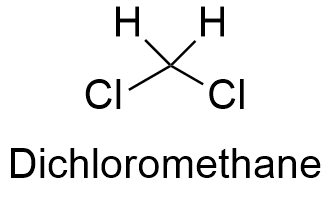 softschools.comdichloromethane formula toxicity occurrence spite found its
softschools.comdichloromethane formula toxicity occurrence spite found its
Liquid Dichloromethane Solvent, Rs 75 /kilogram Plasma Spray Coats | ID
 www.indiamart.comdichloromethane solvent
www.indiamart.comdichloromethane solvent
Dichloromethane 250 Ml Methylene Chloride DCM 99.9% Pure ACS/Lab Grade
 www.ebay.co.ukdcm dichloromethane ml chloride methylene 250ml acs pure lab grade when
www.ebay.co.ukdcm dichloromethane ml chloride methylene 250ml acs pure lab grade when
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
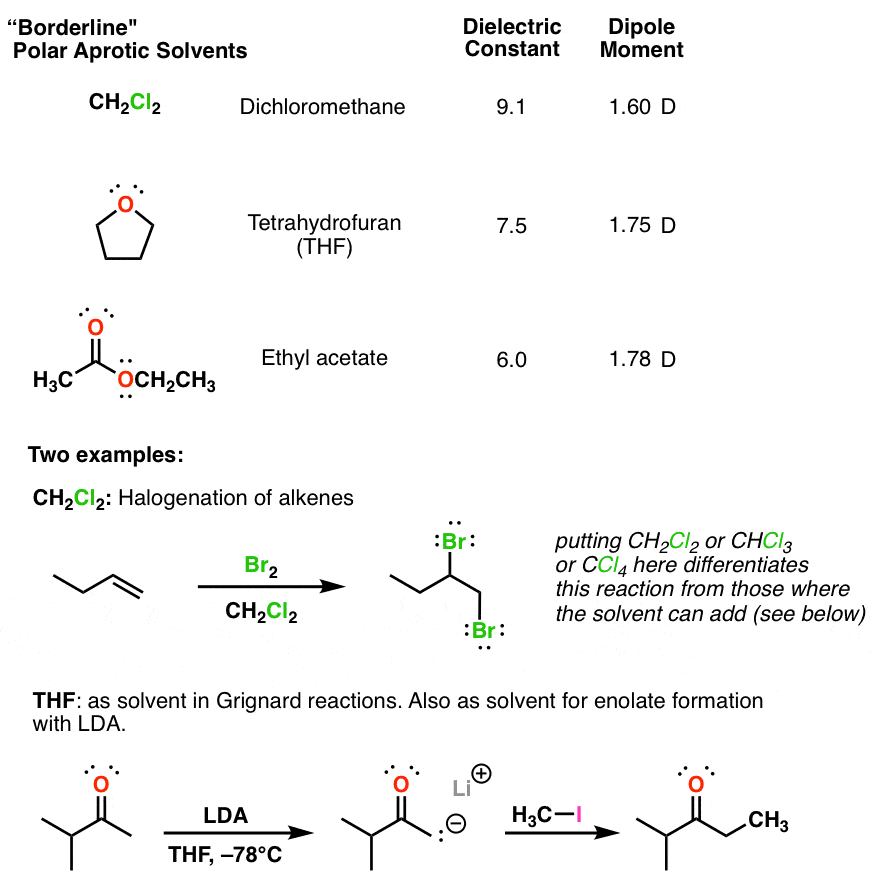 www.masterorganicchemistry.compolar aprotic solvents protic nonpolar thf organic acetate dielectric dichloromethane ethyl non dipole table chemistry reactions constants medium master serve
www.masterorganicchemistry.compolar aprotic solvents protic nonpolar thf organic acetate dielectric dichloromethane ethyl non dipole table chemistry reactions constants medium master serve
Acros Organics AC348460025 Dichloromethane, Extra Dry, With
 www.coleparmer.comdichloromethane acros organics coleparmer
www.coleparmer.comdichloromethane acros organics coleparmer
Dichloromethane acros organics coleparmer. Polar aprotic solvents protic nonpolar thf organic acetate dielectric dichloromethane ethyl non dipole table chemistry reactions constants medium master serve. Dichloromethane formula